GERMINATION.
Not
observed.
 |
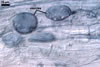 |
In roots of P. lanceolata
|
MYCORRHIZAE.
In Poland, spores of Gl. lamellosum have been associated in the field
with vesicular-arbuscular mycorrhizal roots of Ammophila
arenaria (L.) Link,
Corynephorus
canescens (L.) P. Beauv.,
Elymus
arenarius L.,
and
Hieracium
pilosella L.
(Błaszkowski et al. 2002b). The mycorrhizae formed in single-species cultures
of this fungus with Plantago lanceolata L. as the plant host
consisted of arbuscules, vesicles, as well as intra- and extraradical hyphae.
Arbuscules were irregularly distributed and had fine branches difficult to see.
Vesicles were 21.8-34.3 x 51.7-83.4 µm and usually occurred in conglomerations
highly scattered along roots. Intraradical hyphae were 3.4-5.9 µm wide
and grew parallel to the root axis. These hyphae sometimes formed coils, 18.4-20.3
x 33.8-46.6 µm and Y-shaped branches. Extraradical hyphae were 5.1-6.9
µm wide. In 0.1% trypan blue, arbuscules stained violet grey (18B2-18C3),
vesicles greyish violet (18B3), intraradical hyphae, coils, and extraradical
hyphae greyish violet (18B4, 18B3-18E5, and 18B4-18C6, respectively).
DISTRIBUTION. In
Poland, spores of Gl. lamellosum have so far been found in two field-collected
rhizosphere soils coming from maritime dunes adjacent to Swinoujscie (53o55'N,
14o14'E) and 10 trap cultures with soil-root mixtures that did not harbour
spores of this fungus in the field (Błaszkowski et al. 2002b). The mixtures
came from dunes of Swinoujscie (4 samples), the Vistula Bar (54o21’N,
19o14’E; 2 samples; Błaszkowski et 2002a), and inland dunes of the Bledowska
Desert (50o22'N, 19o34'E; 4 samples; Błaszkowski et al. 2002c).
Glomus
lamellosum has earlier been known only from the sites listed in its original
description (Dalpé et al. 1992). This fungus was associated with Ammophila
breviligulata Fern colonizing a sandy shore of Nottawasaga Bay in Georgian
Bay, Ontario, Canada and sand dunes of Bailey’s harbor, Wisconsin, U.S.A.
NOTES.
The properties most distinguishing spores of Gl. lamellosum are their
thick, hyaline, relatively permanent outermost wall layer (layer 1) producing
a halo in reflected light and the flexible innermost wall layer (layer 3)
staining pinkish white to pastel red in Melzer’s reagent.
The differentiation
of spore wall of Gl. lamellosum comprises successive formation of
discrete layers, beginning from the outermost layer 1. This pattern correspond
with that found in all Glomus spp. investigated ontogenetically to
date (e. g., Błaszkowski and Tadych 1997; Stürmer and Morton 1997).
With age, layer 1 gradually
deteriorates, beginning from its outer surface and, thereby, looses transparency.
However, it is present in most even old spores.
Spore wall layer 2 consists
of tightly adherent, very thin sublayers (laminae). It forms de novo
after the formation of layer 1 is completed.
The innermost spore
wall layer 3 always tightly adheres to the laminate layer 2. Only vigorous
crushing of spores sometimes separates layer 3 from layer 2. However, the
presence of layer 3 most reveals its staining reaction in Melzer’s reagent.
The intensity of staining of layer 3 in Melzer’s reagent varies in different
spores and probably depends on the stage of development of this layer, as
found in, e. g., Entrophospora colombiana Spain & Schenck (Stürmer
and Morton 1999).
The arbuscular mycorrhizal
fungi most resembling Gl. lamellosum are Gl.
claroideum Schenck & Smith, Gl.
clarum Nicolson & Schenck, and Gl. luteum Kennedy, Stutz
& Morton. All these fungi form yellow-coloured spores of a more or less
overlapping size range (Dalpé et al. 1992; Kennedy et al. 1999; Morton
2000; Schenck and Smith 1982; Stürmer and Morton 1997).
The main differences
between the four fungal species are the number and properties of the spore
wall and subtending hyphal wall components. The spore wall of Gl. lamellosum
consists of three layers, whereas that of Gl. claroideum and Gl.
luteum contains four layers (Kennedy et al. 1999; Stürmer and Morton
1997). Glomus lamellosum does not differentiate a mucilaginous outermost
layer staining in Melzer’s reagent as do the letter two species. Additionally,
the outer hyaline layer of Gl. lamellosum spores is much more persistent
than that of the two outer hyaline spore wall layers of Gl. claroideum
and Gl. luteum. It is especially evident in Gl. claroideum,
in which these layers usually are completely sloughed in mature spores.
The most important structure
grouping Gl. lamellosum with Gl. claroideum and Gl.
luteum is the flexible innermost wall layer of their spores. Although
it is phenotypically identical in the three fungi, it stains in Melzer’s
reagent only in Gl. lamellosum (Kennedy et al. 1999; Morton 2000;
Stürmer and Morton 1997). The reactivity of a flexible innermost spore
wall layer infrequently occurs in other Glomus spp. (Morton 2000;
Błaszkowski, pers. observ.). Additionally, the degree of adherence of this
layer to the laminate structural spore wall layer highly differs in these
species: it adheres to the laminate layer in most crushed spores of Gl.
lamellosum, but separates readily from it in the other two species.
Although spores of Gl.
clarum also have a three-layered wall and a halo in reflected light,
those of Gl. lamellosum neither possess the mucilaginous outermost
wall layer (staining in Melzer’s reagent) nor their halo is produced
by a hyaline, laminate wall layer as found in Gl. clarum (Stürmer
and Morton 1997).
Differences between the
four Glomus spp. compared here also reside in the structure of wall
of their subtending hyphae. The wall of subtending hypha of Gl. lamellosum
is two-layered, whereas it consists of three layers in the other fungal species
considered.
Finally, the mycorrhizae
of Gl. lamellosum, Gl. claroideum, and Gl. luteum are
similar: they stain darkly in trypan blue, and vesicles are formed sporadically.
In contrast, despite the mycorrhizal structures of Gl. clarum
that also stain intensively in trypan blue, this fungus frequently forms
intraradical vesicles (Morton 2000).
REFERENCES
Błaszkowski J., Tadych
M. 1997. Glomus multiforum and G. verruculosum, two new
species from Poland. Mycologia 89, 804-811.
Błaszkowski J., Adamska
I., Czerniawska B. 2002a. Arbuscular mycorrhizal fungi (Glomeromycota) of
the Vistula Bar. Acta Mycol. 37, 39-62.
Błaszkowski J., Adamska
I., Madej T. 2002b. Glomus lamellosum (Glomales, Zygomycota), an
arbuscular mycorrhizal fungal species new for Poland and Europe. Mycotaxon
81, 281-292.
Błaszkowski J., Tadych
M., Madej T. 2002c. Arbuscular mycorrhizal fungi (Glomales, Zygomycota) of
the Bledowska Desert, Poland. Acta Soc. Bot. Pol. 71, 71-85.
Dalpé Y., Koske
R. E., Tews L. L. 1992. Glomus lamellosum sp. nov.: a new Glomaceae
associated with beach grass. Mycotaxon 43, 289-293.
Kennedy L. J., Stutz
J. C., Morton J. B. 1999. Glomus eburneum and G. luteum,
two new species of arbuscular mycorrhizal fungi, with emendation of G.
spurcum. Mycologia 91, 1083-1093.
Morton J. B. 2000. International
Culture Collection of Arbuscular and Vesicular-Arbuscular Mycorrhizal Fungi.
West Virginia University.
Schenck N. C., Smith
G. S. 1982. Additional new and unreported species of mycorrhizal fungi (Endogonaceae)
from Florida. Mycologia 74, 77-92.
Stürmer S. L.,
Morton J. B. 1997. Developmental patterns defining morphological characters
in spores of four species in Glomus. Mycologia 89, 72-81.
Stürmer S. L.,
Morton J. B. 1999. Taxonomic reinterpretation of morphological characters
in Acaulosporaceae based on developmental patterns. Mycologia 91, 849-857.